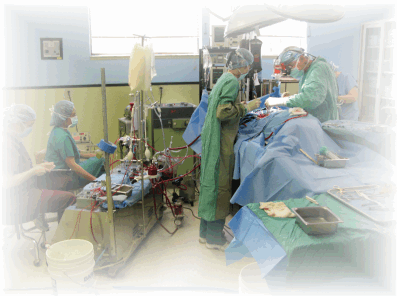
Service Overview
Rapid IACUC turnaround (usually less than 5 days) and study initiation (usually less than 3 weeks).
Facility design allows for strict confidentiality among sponsors doing work simultaneously.
Studies are directly overseen by three qualified Study Directors. Quality Assurance and Quality Control can be provided via in house expertise or from outside subcontractors dependent upon sponsor´s preference. BioSurg professional and technical staff have extensive experience to provide a comprehensive program with regard the testing model, device design and performance, regulatory compliance and concerns and other important aspects of the sponsor´s device or product.
Chronic study animals remain on premises throughout the study rather than being shipped to distant housing facilities to minimize the possible loss of study data.
Animals are observed and/or examined on a two to three times daily basis by California licensed facility veterinarians seven days per week.
Indoor/Outdoor free choice housing and exercise for long term study animals is provided.
Weekend and extended study days available.
Consultation, laboratory and surgical service for the private practice veterinary community involving companion animals.
Consultation services for the research community involving but not limited to: device design, regulatory compliance and/or quality assurance issues, model selection, anesthesia, surgery, analgesia, monitoring critical patient care and animal husbandry.
Cardiopulmonary bypass perfusionist services for the research or private practice community.
Ultrasonography by board certified cardiac sonographer and consultation on use of transesophageal, intracardiac and intravascular (IVUS) imaging and other minimally invasive modalities(i.e. endoscopy, fluoroscopy, laproscopy).
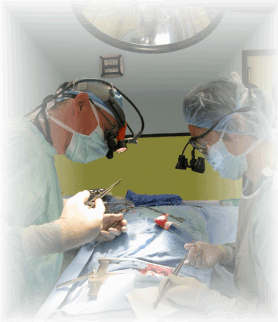
Veterinary Specialty Services
BioSurg, Inc. principals specialize in the development and implementation of studies to evaluate the safety and efficacy of devices and drugs prior to their introduction for human use. The veterinary staff has more than 75 years combined experience in the development, performance and finalizing of studies intended for submission to national and international government regulatory institutions. One principal at BioSurg has nearly 35 years in testing of medical devices and drugs, has extensive research experience and research degree (PhD) in Physiology and Pharmacology, is a boarded veterinary surgeon and for over 25 years was a Professor of veterinary surgery, University of California, Davis. A second principal has extensive laboratory animal and internal medicine experience as well as a comprehensive understanding of USDA, FDA and OLAW regulatory issues dealing with such investigative studies. In addition, boarded veterinary and human consultants in pathology, cardiology and other specialties are utilized to support GLP and feasibility studies.